Lauren R. McCafferty
Department of Emergency Medicine,
University Hospitals Cleveland Medical Center
Point-of-care ultrasound (POCUS) is a focused ultrasound (US) examination performed and interpreted by the clinician at the patient’s bedside to answer a specific clinical question or guide an invasive procedure. Because the clinician can correlate findings with a patient’s signs and symptoms, POCUS can provide valuable diagnostic information to narrow the differential and guide management in real time [1, 2]. Integration of POCUS into clinical practice has been shown to increase diagnostic accuracy and expedite time to diagnosis and treatment, as well as reduce costs and length of stay and improve patient safety and satisfaction [3–8].
POCUS examinations are focused and operator-dependent; therefore, they tend to provide limited information. As such, POCUS does not replace comprehensive radiologist-performed diagnostic studies; CT is overall superior in evaluating patients with an acute abdomen (i.e., sudden onset of severe abdominal pain) [1, 8]. However, because POCUS is portable, noninvasive, radiation-free, and easily repeatable, it is especially valuable in high-acuity settings, where expedient answers and patient stability are a consideration. POCUS may not be the best definitive test, but it is an excellent initial diagnostic tool that can aid in critical decision-making [9]. The scope of POCUS has expanded significantly in recent decades to include a myriad of applications. Among these are targeted evaluations of acute intraabdominal pathology, where rapid recognition and prompt management are key. This includes hemoperitoneum, ectopic pregnancy, abdominal aortic aneurysm (AAA), obstructive uropathy, appendicitis, bowel obstruction, and pneumoperitoneum [9, 10]. This InPractice article provides an overview of the scope and utility of POCUS for the acute abdomen, focusing on commonly used applications in clinical practice.
Free Fluid
Intraperitoneal free fluid is the pathologic accumulation of fluid within the peritoneal cavity. This can result from various disease processes, including traumatic hemorrhage, ruptured ectopic pregnancy, AAA, bowel perforation or obstruction, and ascites. Although many of these pathologies usually warrant additional diagnostic imaging, POCUS is a valuable initial test that can quickly detect free fluid, which can influence workup and management [11].
Free Fluid in Trauma Setting
Evaluating intraperitoneal free fluid in the setting of trauma is among the most well-established uses of POCUS. First described in Europe in the 1970s and adopted in the United States by the 1990s, this examination is now known as “focused assessment with sonography in trauma” (FAST) [12]. The FAST examination includes a quick survey of key areas in the intraperitoneal cavity for free fluid, a sign of hemorrhage, and an indirect indication of organ injury; cardiac and thoracic components are also included. The abdominal component of FAST evaluates the right and left upper quadrants, focusing on the perihepatic and perisplenic spaces, respectively, along with the pelvis [11, 12] (Fig. 1).

The sensitivity and specificity of FAST for detecting intraperitoneal free fluid is 64–98% and 86–100%, respectively [13]. Though not perfect, FAST has greater accuracy compared with physical examination, laboratory tests, and radiography to detect intraabdominal injury. In hemodynamically unstable patients, the diagnostic accuracy increases significantly [14]. Because of its utility, the FAST examination has become the initial screening modality (replacing the previous standard of diagnostic peritoneal lavage) at most trauma centers nationwide and is included in the Advanced Trauma Life Support protocol [15]. A FAST examination is primarily indicated in the setting of blunt trauma but can help triage and prioritize further diagnostic testing and management in cases of penetrating abdominal trauma. A positive FAST—that is, the presence of free fluid—suggests an intraabdominal injury, whereas a negative FAST alone does not obviate additional testing for intraabdominal injury. This is because a FAST examination cannot reliably rule out injuries to solid or hollow organs [13].
The amount of free fluid detected at a specific point in time depends on the rate of accumulation, location, and the patient’s position [9]. Free fluid gravitates to the most dependent area, which is the right upper quadrant (RUQ) in a supine patient [16]. Within the RUQ, the hepatorenal recess (also known as Morison pouch) is a common area of interest; however, the caudal tip of the liver is where fluid tends to collect first. In the left upper quadrant, attention should be directed to the perisplenic area, particularly in the subdiaphragmatic space. In the pelvis, fluid tends to collect posterior to the uterus, known as the pouch of Douglas, in females and in the rectovesicular space or lateral to the bladder in males [17] (Fig. 2).

Despite its recognized utility, the FAST examination has several limitations. In addition to inability to exclude organ injury, it can also be limited by other factors, including operator experience, body habitus, and bowel gas. Free fluid can have varying appearances depending on the type and composition of fluid, and it can change over time. For example, blood is initially anechoic or black, but blood becomes more echogenic as it clots, which makes it difficult to identify and distinguish free fluid from surrounding organs, fat, or other structures. US cannot differentiate the type of fluid (i.e., blood, urine, ascites) and is unable to detect retroperitoneal hemorrhage [11, 12].
Free Fluid in Nontrauma Setting
The FAST examination is highly valuable when evaluating free fluid from nontraumatic hemoperitoneum (such as from ruptured ectopic pregnancy), bowel perforation or obstruction, ascites, and undifferentiated hypotension [11]. When evaluating or managing a patient with ascites, POCUS allows for quantification and distribution of the fluid and can provide real-time procedural guidance for paracentesis at the bedside, which improves success and reduces complications [3]. The other topics are covered separately in the subsequent sections.
Ectopic Pregnancy
Ectopic pregnancy is the leading cause of maternal mortality in the first trimester, and prompt recognition is key [18, 19]. US is the primary imaging modality throughout pregnancy, and the main goals of POCUS, especially in the first trimester, are to identify an intrauterine pregnancy (IUP) and evaluate for free fluid. Confirming an IUP essentially excludes ectopic pregnancy, whereas the absence of an IUP should raise concern for an ectopic pregnancy, especially if the patient has concerning signs or symptoms. Though not a goal of POCUS, it is possible to identify an extrauterine gestational sac containing a yolk sac or fetal pole, which is diagnostic of ectopic pregnancy. Additional nonspecific findings that may also be seen include a complex mass, tubal ring, and free fluid, but their absence does not rule out ectopic pregnancy [11].
In the setting of known or suspected ectopic pregnancy, free fluid in the RUQ is highly concerning for rupture, which can be life-threatening. This finding not only predicts the need for operative intervention, but it significantly expedites the time to diagnosis and definitive management [18–20].
Abdominal Aortic Aneurysm
AAA is a relatively common acute abdominal process with high mortality. A ruptured AAA requires rapid diagnosis and prompt surgical intervention. Clinicians often rely on classic signs and symptoms, including severe abdominal or back pain, syncope, hypotension, or a pulsatile abdominal mass, but these have poor sensitivity [21]. CTA is the preferred imaging study for ruptured AAA, and US is a well-established and validated screening modality [22].
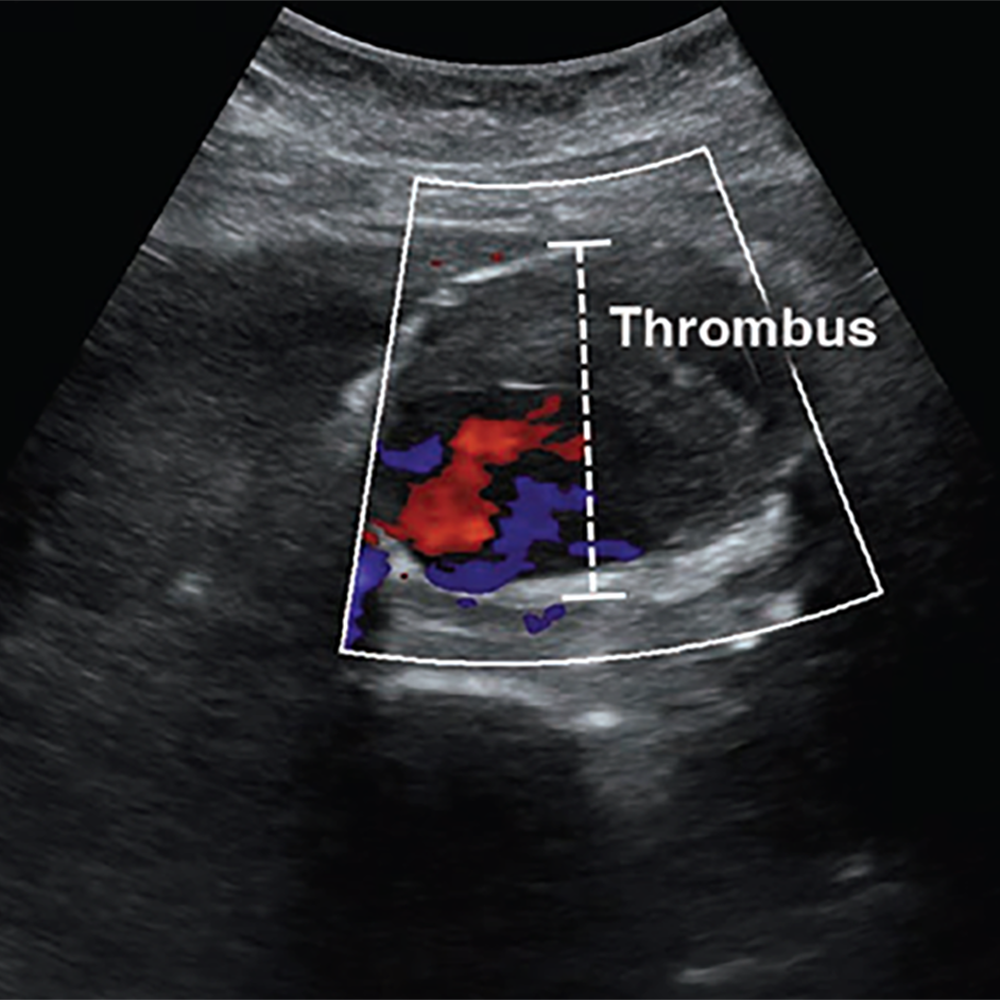
POCUS has excellent ability to detect AAA, which is defined by a diameter exceeding 3 cm when measured from outer wall to outer wall (Fig. 3).

A systematic review and meta-analysis found POCUS to have a sensitivity of 99% and specificity of 98%, when performed by emergency medicine physicians [23]. Although POCUS can accurately determine the presence or absence of AAA, the ability to detect signs of rupture is poor, which is largely due to the limited ability to visualize the retroperitoneum. Findings that indicate rupture include deformation of aneurysmal shape, heterogeneity or focal discontinuity of the intraluminal thrombus, focal disruption of the outer wall, hypoechoic areas in the paraaortic region, and hemoperitoneum [24].
POCUS is a great initial imaging option for suspected AAA, especially in the emergency department (ED), but adequate visualization may be limited by body habitus or bowel gas. The latter can be mitigated by graded compression, whereby slow, sustained pressure is applied to the abdomen to displace loops of bowel (and associated intraluminal gas) to allow better visualization of underlying structures. Like the FAST examination, AAA assessment is one of the core POCUS applications and is a required part of residency training for nonradiology specialties [10].
Cholelithiasis and Cholecystitis
Cholelithiasis and cholecystitis are common biliary pathologies characterized by gallstones in the gallbladder and inflammation of the gallbladder, respectively. US is the reference standard for diagnosis. Biliary POCUS is well established for identifying cholelithiasis or cholecystitis [10].
Sonographically, gallstones typically appear as a hyperechoic structure with distinct posterior shadowing. Imaging findings of cholecystitis include thickened gallbladder wall, pericholecystic fluid, and sonographic Murphy sign, in addition to sludge or gallstones in most cases [11] (Fig. 4).

Like other abdominal POCUS examinations, body habitus and bowel gas can be limiting. For the latter, having the patient sit upright or move into a left lateral decubitus position can often help move the bowel loops away from the gallbladder to improve visualization. Additional pitfalls include misidentification of anatomy or mistaking normal physiologic changes with pathologic findings, such as a contracted gallbladder [11, 25].
Despite these limitations, emergency physician–performed POCUS for cholelithiasis and cholecystitis has sensitivity and specificity comparable to that of radiologist-performed US [25, 26]. Biliary POCUS alone has been shown to reliably inform surgical decision-making and can reduce length of stay in the ED [27, 28]; however, routine adoption in clinical practice has been limited. Although POCUS does not replace comprehensive imaging, it is a safe, efficient, and reliable diagnostic option for cholelithiasis and cholecystitis.
Obstructive Uropathy
Obstructive uropathy can result from intrinsic or extrinsic obstruction of the urinary tract system and can be unilateral or bilateral, depending on the etiology. Renal colic commonly results from nephrolithiasis, which can lead to hydronephrosis when obstruction occurs. CT is the preferred imaging study for suspected nephrolithiasis, but POCUS is appropriate for initial imaging examination [11].
The focus of renal POCUS is to detect signs of obstruction (i.e., hydronephrosis and urinary retention) rather than identifying the stone, itself. POCUS is less sensitive, but more specific for nephrolithiasis compared with CT and has test characteristics comparable to those of traditional US. Use of POCUS is associated with shorter length of stay in the ED [29]. POCUS is highly specific for nephrolithiasis with moderate hydronephrosis, but less accurate in cases of mild or no hydronephrosis [30] (Fig. 5).

Advanced signs of obstruction are associated with larger stones, which often require surgical intervention, rather than conservative management [31]. For younger patients in whom uncomplicated renal colic is suspected, POCUS is a favorable initial imaging modality and perhaps the only imaging study needed. In patients with less typical signs and symptoms, POCUS can influence clinical suspicion and help inform the need for further imaging [32].
Appendicitis
Appendicitis is the most common surgical emergency worldwide and can be complicated by perforation, which occurs in approximately 20% of cases [33]. Along with clinical and laboratory findings, multiple imaging studies play a role in diagnosing appendicitis. CT may be the most accurate, but radiologist-performed US is well established and especially favorable in children in whom radiation exposure is a concern [34, 35]. POCUS has emerged as a promising diagnostic tool with relatively high sensitivity and specificity for appendicitis [34].
Characteristic sonographic findings include target sign in the short axis, blind-ended pouch in the long axis, lack of compressibility, diameter greater than 6 mm, wall thickness greater than 3 mm, appendicolith, and hypervascularity. Indirect findings suggestive of appendicitis include periappendiceal free fluid or abscess, hyperechoic mesenteric fat, enlarged mesenteric lymph nodes, increased peritoneal thickness, and signs of small bowel obstruction (SBO) [35] (Fig. 6).


Benefits of POCUS examination include lack of radiation exposure, lower costs, and ability to help prioritize radiology studies or expedite surgical consult. It may be particularly useful in centers where radiologist-performed US is not continually available. Visualizing the appendix is often limited by body habitus, pain, retrocecal location, and operator skill and experience. In addition, because a normal appendix is often difficult to visualize, this may be a more challenging POCUS study to learn [9, 34]. POCUS is a promising adjunct diagnostic tool, but it has not been extensively studied as a stand-alone test for appendicitis [34].
Bowel Obstruction
Bowel obstruction is a common acute abdominal process that needs timely diagnosis and management to avoid complications, such as ischemia, perforation, and necrosis. Obstruction is defined by impaired flow of bowel contents with varying degrees, ranging from partial to complete obstruction [36]. CT is commonly used to diagnose SBO but can be time-consuming, expensive, and involve radiation exposure. Radiography is often used as an initial imaging study, but sensitivity and specificity are poor and outperformed by POCUS [37]. A systematic review and meta-analysis found POCUS to have a high sensitivity and specificity for diagnosing SBO, rivaling that of CT, with the added benefit of saving time and potentially radiation [38]. The high diagnostic accuracy of POCUS is primarily for complete obstructions; POCUS is less reliable for partial obstruction [39]. Like other POCUS applications, evaluating SBO is highly operator-dependent. Although it may be easy to learn and demonstrate competency, US fellowship training is associated with significantly increased diagnostic accuracy [37–40].
Sonographic findings of SBO include dilated bowel with a diameter of greater than 2.5 cm, fluid-filled loops of bowel, decreased peristaltic movement, increased wall thickness, prominent valvulae conniventes, and a collapsed colonic lumen [40, 41] (Fig. 7).

Free fluid in the peritoneal cavity is associated with higher-grade obstruction and predicts need for operative intervention [42]. A transition point is often difficult to visualize, but POCUS can locate and identify the potential cause of obstruction, such as hernia, intussusception, masses, and signs of ischemia [43]. Evaluating SBO is not a core application of POCUS; it is a suitable modality for initial imaging evaluation and early management of SBO.
Pneumoperitoneum
Pneumoperitoneum is free air in the peritoneal cavity [44]. Free air suggests perforation of a hollow viscus organ, which can result from weakening of the bowel wall, ischemia, foreign body, bowel obstruction, or infection and has high morbidity and mortality [45]. CT is the imaging study of choice. Radiography is often used as an initial diagnostic study for pneumoperitoneum, but sensitivity is generally poor, especially when the amount of air is small. An upright lateral view has greater sensitivity compared with posterior-anterior or supine views, but this is not always possible, especially in critically ill patients [45, 46]. POCUS has proven value as an initial diagnostic test that can expedite recognition and timely management of pneumoperitoneum [44, 47]. US cannot identify the site or extent of perforation like CT, but POCUS has greater sensitivity than radiography with comparable specificity and PPV [48].
On US, free air is evaluated by focusing on the least dependent areas in the peritoneal cavity, where the air tends to migrate. In a supine patient, air moves anteriorly toward the interface between the peritoneal cavity and anterior abdominal wall and is often best identified in the RUQ over the liver [9, 49]. The highly reflective surface of air produces increased echogenicity of the peritoneal line, which is referred to as the enhanced peritoneal stripe sign. Often accompanying this is reverberation artifact, consisting of repeating hyperechoic horizontal lines directly below the enhanced peritoneal line. “Dirty” shadowing may be seen, obscuring the underlying organs (Fig. 8A). Because air can move freely within the peritoneal cavity, these findings change with patient position, known as shifting phenomenon [9, 47, 49]. Similarly, the scissors maneuver can help detect free air and visualize its movement. This technique consists of applying pressure with the probe over the liver to displace the free air and associated artifacts, making these findings less prominent. When the compression is released, the free air returns and associated artifacts become visible again [50].
An important potential pitfall is mistaking free air for intraluminal bowel gas. To differentiate between the two, it helps to focus on the hepatic region where bowel gas is minimal and consider position change or compression to help further identify pneumoperitoneum. Free air moves independent of respiration and peristalsis, unlike intraluminal air in the bowel [9, 47].
Gastric contents in the peritoneal cavity may accompany pneumoperitoneum in cases of bowel perforation. On US, this will appear as free fluid with echogenic debris in the dependent areas of the abdomen [9, 47, 49] (Fig. 8).


POCUS is a valuable diagnostic tool that can be applied at the bedside to quickly answer focused clinical questions. With a broad and continually expanding scope, POCUS is used by many specialties and is incorporated in medical education and training. Although POCUS does not replace comprehensive imaging in patients with an acute abdomen, it has value as an initial imaging modality that can rapidly provide key diagnostic information. POCUS can influence clinical suspicion, guide decision-making, and expedite the diagnosis and treatment by enabling providers to correlate clinical and imaging findings with the patient in real time.
References
- Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med 2011; 364:749–757
- Jang T, Chauhan V, Cundiff C, Kaji AH. Assessment of emergency physician-performed ultrasound in evaluating nonspecific abdominal pain. Am J Emerg Med 2014; 32:457–460
- Peabody CR, Mandavia D. Deep needle procedures: improving safety with ultrasound visualization. J Patient Saf 2017; 13:103–108
- Roxas R, Gallegos L, Bailitz J. Rapid detection of aortic occlusion with emergency ultrasonography. Ann Emerg Med 2011; 58:21–23
- Lindelius A, Torngren S, Nilsson L, Pettersson H, Adami J. Randomized clinical trial of bedside ultrasound among patients with abdominal pain in the emergency department: impact on patient satisfaction and health care consumption. Scand J Trauma Resusc Emerg Med 2009; 17:60
- Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med 2014; 46:46–53
- Durgun Y, Yurumez Y, Guner NG, Aslan N, Durmus E, Kahraman Y. Abdominal pain management and point-of-care ultrasound in the emergency department: a randomised, prospective, controlled study. J Coll Physicians Surg Pak 2022; 32:1260–1265
- Laméris W, van Randen A, van Es HW, et al. Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ 2009; 338:b2431
- Abu-Zidan FM, Cevik AA. Diagnostic point-of-care ultrasound (POCUS) for gastrointestinal pathology: state of the art from basics to advanced. World J Emerg Surg 2018; 13:47
- [No authors listed]. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med 2017; 69:e27–e54
- Ma O, Mateer JR, Reardon RF, Joing SA, eds. Ma and Mateer’s emergency ultrasound, 3rd ed. McGraw Hill, 2014
- Bloom BA, Gibbons RC. Focused assessment with sonography for trauma. StatPearls Publishing, 2023
- Körner M, Krötz MM, Degenhart C, Pfeifer KJ, Reiser MF, Linsenmaier U. Current role of emergency US in patients with major trauma. RadioGraphics 2008; 28:225–242
- Nishijima DK, Simel DL, Wisner DH, Holmes JF. Does this adult patient have a blunt intra-abdominal injury? JAMA 2012; 307:1517–1527
- Boulanger BR, Kearney PA, Brenneman FD, Tsuei B, Ochoa J. Utilization of FAST (focused assessment with sonography for trauma) in 1999: results of a survey of North American trauma centers. Am Surg 2000; 66:1049–1055
- Rozycki GS, Ochsner MG, Feliciano DV, et al. Early detection of hemoperitoneum by ultrasound examination of the right upper quadrant: a multicenter study. J Trauma 1998; 45:878–883
- Lobo V, Hunter-Behrend M, Cullnan E, et al. Caudal edge of the liver in the right upper quadrant (RUQ) view is the most sensitive area for free fluid on the FAST exam. West J Emerg Med 2017; 18:270–280
- Rodgerson JD, Heegaard WG, Plummer D, Hicks J, Clinton J, Sterner S. Emergency department right upper quadrant ultrasound is associated with a reduced time to diagnosis and treatment of ruptured ectopic pregnancies. Acad Emerg Med 2001; 8:331–336
- Moore C, Todd WM, O’Brien E, Lin H. Free fluid in Morison’s pouch on bedside ultrasound predicts need for operative intervention in suspected ectopic pregnancy. Acad Emerg Med 2007; 14:755–758
- Stone BS, Muruganandan KM, Tonelli MM, Dugas JN, Verriet IE, Pare JR. Impact of point-of-care ultrasound on treatment time for ectopic pregnancy. Am J Emerg Med 2021; 49:226–232
- Fernando SM, Tran A, Cheng W, et al. Accuracy of presenting symptoms, physical examination, and imaging for diagnosis of ruptured abdominal aortic aneurysm: systematic review and meta-analysis. Acad Emerg Med 2022; 29:486–496
- Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999; 17:472–475
- Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med 2013; 20:128–138
- Catalano O, Siani A. Ruptured abdominal aortic aneurysm: categorization of sonographic findings and report of 3 new signs. J Ultrasound Med 2005; 24:1077–1083
- Ross M, Brown M, McLaughlin K, et al. Emergency physician-performed ultrasound to diagnose cholelithiasis: a systematic review. Acad Emerg Med 2011; 18:227–235
- Summers SM, Scruggs W, Menchine MD, et al. A prospective evaluation of emergency department bedside ultrasonography for the detection of acute cholecystitis. Ann Emerg Med 2010; 56:114–122
- Hilsden R, Mitrou N, Hawel J, Leeper R, Thompson D, Myslik F. Point of care biliary ultrasound in the emergency department (BUSED) predicts final surgical management decisions. Trauma Surg Acute Care Open 2022; 7:e000944
- Blaivas M, Harwood RA, Lambert MJ. Decreasing length of stay with emergency ultrasound examination of the gallbladder. Acad Emerg Med 1999; 6:1020–1023
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med 2014; 371:1100–1110
- Wong C, Teitge B, Ross M, Young P, Robertson HL, Lang E. The accuracy and prognostic value of point-of-care ultrasound for nephrolithiasis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med 2018; 25:684–698
- Goertz JK, Lotterman S. Can the degree of hydronephrosis on ultrasound predict kidney stone size? Am J Emerg Med 2010; 28:813–816
- Moore CL, Carpenter CR, Heilbrun ME, et al. Imaging in suspected renal colic: systematic review of the literature and multispecialty consensus. Ann Emerg Med 2019; 74:391–399
- Körner H, Söndenaa K, Söreide J, et al. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg 1997; 21:313–317
- Fields JM, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med 2017; 24:1124–1136
- Mostbeck G, Adam EJ, Nielsen MB, et al. How to diagnose acute appendicitis: ultrasound first. Insights Imaging 2016; 7:255–263
- Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology 2015; 275:332–342
- Jang TB, Schindler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J 2011; 28:676–678
- Gottlieb M, Peska GD, Pandurangadu AV, Nakitende D, Takhar S, Seethala RR. Utilization of ultrasound for the evaluation of small bowel obstruction: a systematic review and meta-analysis. Am J Emerg Med 2018; 36:234–242
- Pourmand A, Dimbil U, Drake A, Shokoohi H. The accuracy of point-of-care ultrasound in detecting small bowel obstruction in emergency department. Emerg Med Int 2018; 2018:3684081
- Becker BA, Lahham S, Gonzales MA, et al. A prospective, multicenter evaluation of point-of-care ultrasound for small-bowel obstruction in the emergency department. Acad Emerg Med 2019; 26:921–930
- Hefny AF, Corr P, Abu-Zidan FM. The role of ultrasound in the management of intestinal obstruction. J Emerg Trauma Shock 2012; 5:84–86
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol 2004; 50:5–14
- Radonjić T, Popović M, Zdravković M, et al. Point-of-care abdominal ultrasonography (POCUS) on the way to the right and rapid diagnosis. Diagnostics (Basel) 2022; 12:2052
- Bacci M, Kushwaha R, Cabrera G, Kalivoda EJ. Point-of-care ultrasound diagnosis of pneumoperitoneum in the emergency department. Cureus 2020; 12:e8503
- Langell JT, Mulvihill SJ. Gastrointestinal perforation and the acute abdomen. Med Clin North Am 2008; 92:599–625
- Woodring JH, Heiser MJ. Detection of pneumoperitoneum on chest radiographs: comparison of upright lateral and posteroanterior projections. AJR 1995; 165:45–47
- Jones R. Recognition of pneumoperitoneum using bedside ultrasound in critically ill patients presenting with acute abdominal pain. Am J Emerg Med 2007; 25:838–841
- Chen SC, Yen ZS, Wang HP, Lin FY, Hsu CY, Chen WJ. Ultrasonography is superior to plain radiography in the diagnosis of pneumoperitoneum. Br J Surg 2002; 89:351–354
- Shokoohi H, Boniface KS, Abell BM, Pourmand A, Salimian M. Ultrasound and Perforated viscus, dirty fluid, dirty shadows, and peritoneal enhancement. Emerg (Tehran) 2016; 4:101–105
- Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound 2004; 32:381–3
The opinions expressed in InPractice magazine are those of the author(s); they do not necessarily reflect the viewpoint or position of the editors, reviewers, or publisher.