Sadia R. Qamar, Ramin Hamidizadeh, Samad Shah, Patrick McLaughlin, Anto Sedlic, Savvas Nicolaou
Department of Radiology
University of British Columbia
Memoona Mian
Department of Radiology
University of Arkansas for Medical Sciences
Prompt and early diagnosis is vital for timely treatment of traumatic cardiac emergencies. Myocardial rupture is a rare cause of immediate death after blunt cardiac trauma, with only 0.3–1.1% of patients with trauma reaching the emergency department (ED) [1]. Pericardial tears caused by deceleration forces or rib cage fractures are uncommon after blunt chest trauma, with a frequency of 0.3–0.5%. Rarely, valvular dysfunction can be seen due to an abrupt raised intracardiac pressure against a closed valve resulting from sudden rise in intraabdominal pressure translating into the heart causing valve cusp avulsion or tear [2]. Penetrating trauma can result in pericardial injuries further complicated by life-threatening conditions including partial or complete transdefect cardiac herniation or luxation with a mortality rate as high as 67%.
Plain radiographs will show pneumopericardium, hydrothorax or hemothorax, and mediastinal hematoma. Echocardiography will show abnormal valve function; wall motion abnormalities with decreased left ventricular ejection fraction; and pericardial effusion, signs of cardiac tamponade, or both. CT will show pneumopericardium, pericardial effusion, pericardial or myocardial laceration or rupture, cardiac herniation or luxation with associated SVC obstruction or right heart strain, valvular cusp avulsion or tears, coronary artery dissection or rupture, and associated rib cage fractures, retained foreign bodies, bullet fragments, and wound tracks [3] (Fig. 1).


Fig. 1—29-year-old man with gunshot wound. Left, Axial four-chamber cardiac CT image shows bullet fragment (arrow) abutting left ventricular side wall at mid cardiac level without myocardial penetration. Right, Mid ventricle short-axis color-coded functional cardiac CT image depicts reduced perfusion (white arrow) consistent with myocardial contusion. Black arrow indicates bullet fragment.
Imaging Pearls and Pitfalls in Cardiac Trauma
Any pericardial effusion detected in the acute trauma setting is presumed to be hemopericardium until proven otherwise. CT provides valuable information about the possible nature of pericardial effusions on the basis of the attenuation measurements of the collection. Coronary artery injuries are rare (in less than 2% of chest trauma cases), with left anterior descending artery being most commonly injured [4]. Penetrating cardiac trauma can result in pericardial injuries, which can result in partial or complete transdefect cardiac herniation or luxation with mortality up to 70% [5]. Portable supine studies in ED are suboptimal with overlying artifacts, which limits evaluation. TEE is invasive and difficult to perform in patients with acute craniocervical injuries. Cardiac MRI in trauma is primarily useful as a problem-solving tool after patients are admitted, especially to delineate the extent of myocardial contusion, regional infarction, wall motion abnormality, and valvular dysfunction.
Limitations of MDCT
First, CT involves use of ionizing radiation which increase the radiation exposure in the population. Second, the quality of MDCT images suffers with fast heart rate and high calcium burden. Finally, the patients with arrhythmias, ectopy or misregistration ECG artifacts degrade the image quality and limit evaluation. Optimizing techniques should be incorporated to counter these limiting factors.
Reduction of Radiation Dose
Radiation dose can be reduced with a prospective ECG-gated technique with narrow window acquisition, ECG tube current modulation, and limited pulse windows; tube voltage reduction based on body mass index; automated tube voltage reduction based on topogram attenuation profile; adaptive collimation limiting helical over spiral scanning; and iterative reconstructive techniques to reduce noise and ultimately reduce dose.
Optimizing Quality of Cardiac CT
Several steps can be taken to optimize the quality of cardiac CT studies. A heart rate of less than 65 beats/min can be achieved by administering 5–20 mg of β-blocker (metoprolol) IV or 50–100 mg by mouth 1 hour before the CT. Lowering the heart rate widens diastole and decreases beat-to-beat variability. Coronary arterial dilatation for optimal visualization can be achieved by administering 0.4–0.8 mg of nitroglycerin sublingually 5 minutes before contrast injection. Reconstruction algorithms can be used to reduce beam-hardening artifacts from iodine that mimic ischemia (Fig. 2).

Fig. 2—CT images show beam-hardening correction (left) and utility of B23 kernel (right) as it reduces beam-hardening artifact from iodine in left ventricle and thoracic aorta, affecting posterior inferior aspect of left ventricle wall mimicking infarct.
Edge-enhancing reconstruction algorithms can be used to reduce noise caused by extensive coronary calcifications or coronary stents.
Emerging Applications and Outlook
Coronary Atherosclerotic Plaque Characterization
The rationale behind growing efforts to accurately characterize a vulnerable, predominantly lipid-rich, plaque is its grave association with ACS and SCD. Novel attenuation-based application of dual-energy CT (DECT) has shown promising results when correlated with histologic findings. Spectral attenuation curves for material characterization are generated using attenuation values of a specific material for each and every monochromatic energy ranging from 40 to 140 keV [6]. Lipid-rich atherosclerotic plaques share the known attenuation curve of fat, in which attenuation decreases with lower monochromatic energy, thus differentiating lipid-rich plaques from fibrous plaques [7].
CT-Derived Fractional Flow Reserve
Coronary blood-flow volume effectively provides an estimation of lesion-specific ischemia. Recent vigorous advancements in digital analysis of fluid dynamics allow noninvasive assessment of coronary flow on the basis of mathematic models. CT-derived fractional flow reserve (FFR) calculates lesion-specific FFR using static coronary CT data without additional radiation or modification in image acquisition protocols. Studies have found that CT FFR shows 90% sensitivity and nearly 83% specificity for lesions with moderate stenosis causing ischemia [38]. A multicenter prospective trial showed 73% specificity and 90% sensitivity for CT FFR in diagnosing obstructive CAD compared with conventional angiographic FFR [8].
CT Myocardial Perfusion and Viability
Myocardial perfusion is one of the most important prognostic indicators for patient outcome and management of CAD. CT myocardial blood pool analysis using myocardial iodine content is a promising dynamic technology. DECT color-coded iodine maps permit sensitive detection of myocardial perfusion by depicting myocardial blood pooling [9]. The perfused myocardium takes up iodine, but no iodine uptake is seen in the infarcted myocardium (Fig. 3). Assessment of myocardial viability predicts successful revascularization therapy.
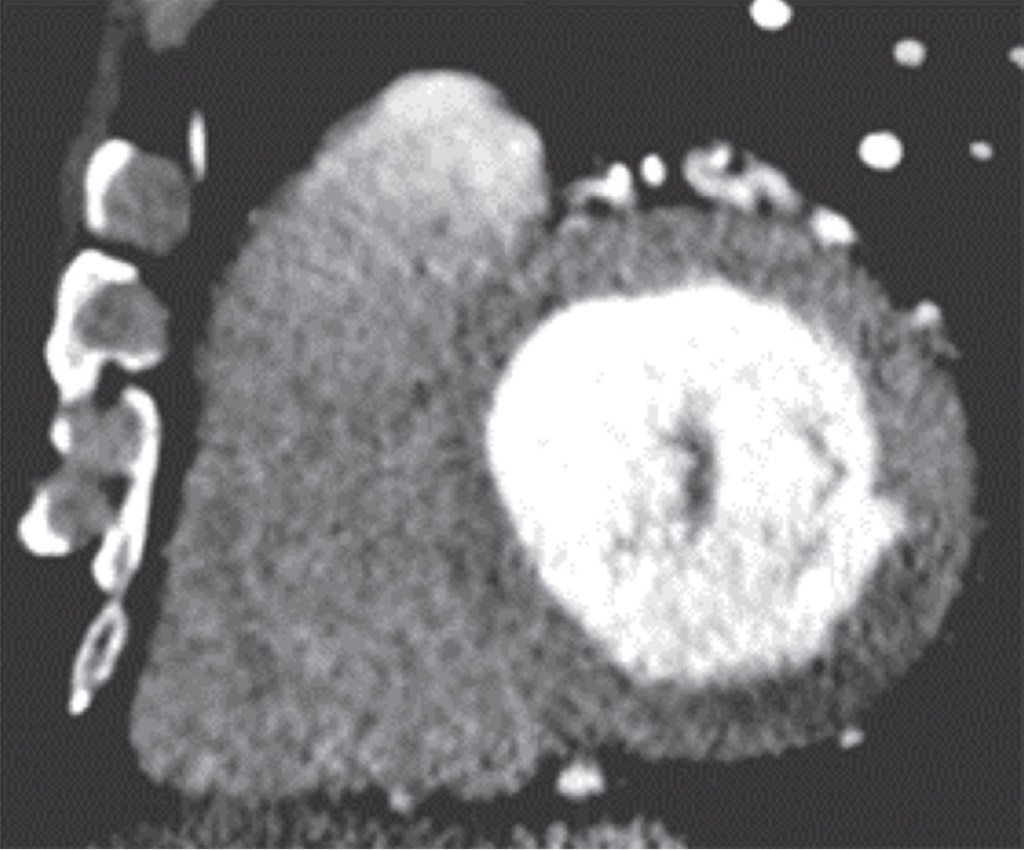

Fig. 3—Myocardial ischemia in 51-year-old man. Left and right, Mid ventricle short-axis cardiac CT image (left) and iodine perfusion map (right) show decreased subendocardial iodine uptake (arrows) in inferior basal ventricle, suggesting perfusion defect consistent with myocardial ischemia.
MDCT is a viable, reliable, and potentially effective imaging modality in evaluation of coronary and noncoronary cardiac emergencies. Cardiac CT efficiently rules out CAD in patients with low to intermediate risk who present with acute chest pain in the ED and accurately predicts midterm adverse outcome. With integration of innovative applications like morphologic plaque characterization, coronary FFR and myocardial perfusion, cardiac CT will be able to offer unprecedented benefits, ranging from triage to treatment decisions in the ED.
References
- Mirvis SE. Imaging of acute thoracic injury: the advent of MDCT screening. Semin Ultrasound CT MR 2005; 26:305–331
- Farhataziz N, Landay MJ. Pericardial rupture after blunt chest trauma. J Thorac Imaging 2005; 20:50–52
- Sohn JH, Song JW, Seo JB, et al. Pericardial rupture and cardiac herniation after blunt trauma: a case diagnosed using cardiac MRI. Br J Radiol 2005; 78:447–449
- Bruschi G, Agati S, Iorio F, Vitali E. Papillary muscle rupture and pericardial injuries after blunt chest trauma. Eur J Cardiothorac Surg 2001; 20:200–202
- Prêtre R, Chilcott M. Blunt trauma to the heart and great vessels. N Engl J Med 1997; 336:626–632
- Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol 2001; 21:1618–1622
- Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012; 308:1237–1245
- Yoon YE, Choi JH, Kim JH, et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc Imaging 2012; 5:1088–1096
- Han R, Sun K, Lu B, Zhao R, Li K, Yang X. Diagnostic accuracy of coronary CT angiography combined with dual-energy myocardial perfusion imaging for detection of myocardial infarction. Exp Ther Med 2017; 14:207–213

